Have you ever wondered why things stick together? Why does water always seem to have that special bond? Well, the answer lies in the fascinating topic of atoms and molecules, the building blocks of everything around us. Atoms form molecules through a process called chemical bonding. The fundamental reason behind this is the tendency of atoms to achieve a more stable and lower energy state by forming bonds with other atoms. If you're eager to explore the secrets behind these tiny particles' extraordinary dance, there's no better place to embark on your journey than Miracle Learning Centre, where chemistry tuition becomes an adventure. Let's delve into the mysteries of how atoms give rise to the formation of molecules.
Atoms -The Building Blocks of Matter
Let's start with the basics. Atoms are like the LEGO bricks of the universe. They're the smallest units of an element and have a central nucleus surrounded by electrons whizzing around. Each atom has a specific number of electrons, protons, and neutrons.
Outer Electron Shells - The Key Players
The outermost layer of an atom, known as the electron shell, is crucial in chemical bonding. Atoms are happiest when their outer electron shells are full. Just like we feel complete with a full plate of our favorite food, atoms strive for a full outer shell to be stable.
Different Types of Chemical Bonds
There are several types of chemical bonds, but the two most common are covalent bonds and ionic bonds.
1. Covalent Bonds
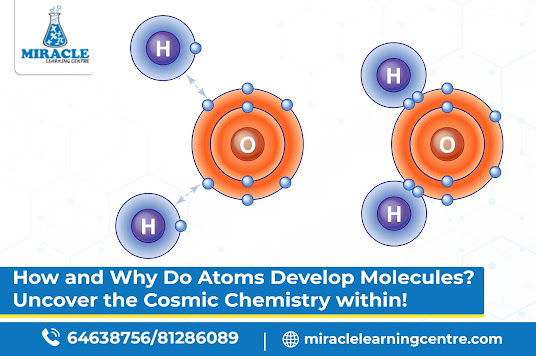
One way atoms achieve stability is through covalent bonding. Picture two atoms holding hands and sharing electrons like a friendship bracelet. They share to ensure that both end up with a full outer electron shell. Water (H2O) is a fantastic example: two hydrogen atoms join forces with one oxygen atom through covalent bonds, creating a molecule essential for life.
2. Ionic Bonds
Sometimes, atoms play a game of give and take, leading to ionic bonding. Imagine one atom saying, "I'll give you an electron," and the other saying, "Great! Now I have it." This exchange results in charged particles called ions. Sodium chloride (NaCl), or table salt, is a classic example of ionic bonding. Sodium gives an electron to chlorine, and they become a salty duo.
The Quest for Stability - Noble Gas Envy
Why do atoms bother with all this bonding? It's all about achieving a stable, low-energy state. Atoms aim to mimic the noble gases (like helium and neon) that boast full outer electron shells and are super stable. By forming bonds, atoms get closer to achieving this noble gas-like stability.
Why to Choose Miracle Learning Centre?
As one of the best chemistry tuition in Singapore, Miracle Learning Centre understands the importance of making learning fun and engaging, especially for kids. With experienced chemistry tutors and interactive lessons, they turn complex chemistry concepts into exciting adventures. Imagine learning about atoms and molecules as if you're exploring a magical place where every particle has a story to tell.


No comments:
Post a Comment